Case Summary
Roche and Chugai Pharma made a joint voluntary admission about a media release which they had issued on 13 June 2008. The media release related to the presentation of new clinical data on tocilizumab, a biologic therapy currently under consideration for marketing authorization by the US and European regulatory authorities for the management of rheumatoid arthritis. These data were presented at the recent European League Against Rheumatism (EULAR) meeting.
Following discussions with Wyeth it had become apparent that the headline claims 'New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis' and 'This new data, presented today at the European League Against Rheumatism (EULAR) meeting in Paris, makes tocilizumab the first and only biologic therapy to have achieved superiority over MTX [methotrexate]' within the media release might be considered factually incorrect when read alone and therefore might be in breach of the Code.
During inter-company dialogue Wyeth had asked for a corrective statement to be published in scientific journals. However, as tocilizumab was currently unlicensed Roche and Chugai considered that such a statement would potentially be in breach of the Code. Therefore inter-company dialogue had been unsuccessful and thus Roche and Chugai had decided that a voluntary submission to the Authority was the only appropriate course of action.
The Constitution and Procedure provided that the Director should treat a voluntary admission as a complaint if it related to a potentially serious breach of the Code or if the company failed to take appropriate action to address the matter. Issuing a potentially misleading press release was a serious matter and the admission was accordingly treated as a complaint.
The detailed response from Roche and Chugai is given below.
The Panel considered that the heading, 'New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis' was a strong unqualified claim. The first paragraph explained that the current standard of care was methotrexate. The Panel noted the companies' submission that other biologic therapies had shown superiority but unlike tocilizumab not across all American College of Rheumatology (ACR) measures. Superiority had not been uniformly shown in this regard at 6 months and it was this point that was intended to be conveyed inthe media release. The Panel was concerned about the general claims for superiority. The media release also contained the claim 'No previous biologic therapy has demonstrated superiority compared to MTX' which was not so. The Panel noted that the media release had been sent to UK national and medical media. The product was not authorized in the UK and the media release was extremely positive; it used 'novel', 'innovative' and 'most exciting' to describe the product. The Panel considered that the media release was not factual and that the results of a clinical study had not been presented in a balanced way. The media release would raise unfounded hopes of successful treatment. Thus the Panel ruled a breach of the Code.
The Panel considered that given its comments above high standards had not been maintained. A further breach of the Code was ruled.
Roche Products Ltd and Chugai Pharma UK Ltd made a joint voluntary admission about a media release (ref PRX3158) concerning tocilizumab issued on 13 June.
Claims 'New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis' and 'This new data, presented today at the European League Against Rheumatism (EULAR) meeting in Paris, makes tocilizumab the first and only biologic therapy to have achieved superiority over MTX [methotrexate]'
CASES AUTH/2154/8/08 and AUTH/2155/8/08 VOLUNTARY ADMISSION BY ROCHE and CHUGAI
Tocilizumab media release
Roche and Chugai Pharma made a joint voluntary admission about a media release which they had issued on 13 June 2008. The media release related to the presentation of new clinical data on tocilizumab, a biologic therapy currently under consideration for marketing authorization by the US and European regulatory authorities for the management of rheumatoid arthritis. These data were presented at the recent European League Against Rheumatism (EULAR) meeting.
Following discussions with Wyeth it had become apparent that the headline claims ‘New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis’ and ‘This new data, presented today at the European League Against Rheumatism (EULAR) meeting in Paris, makes tocilizumab the first and only biologic therapy to have achieved superiority over MTX [methotrexate]’ within the media release might be considered factually incorrect when read alone and therefore might be in breach of the Code.
During inter-company dialogue Wyeth had asked for a corrective statement to be published in scientific journals. However, as tocilizumab was currently unlicensed Roche and Chugai considered that such a statement would potentially be in breach of the Code. Therefore inter-company dialogue had been unsuccessful and thus Roche and Chugai had decided that a voluntary submission to the Authority was the only appropriate course of action.
The Constitution and Procedure provided that the Director should treat a voluntary admission as a complaint if it related to a potentially serious breach of the Code or if the company failed to take appropriate action to address the matter. Issuing a potentially misleading press release was a serious matter and the admission was accordingly treated as a complaint.
The detailed response from Roche and Chugai is given below.
The Panel considered that the heading, ‘New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis’ was a strong unqualified claim.
The first paragraph explained that the current standard of care was methotrexate. The Panel noted the companies’ submission that other biologic therapies had shown superiority but unlike tocilizumab not across all American College of Rheumatology (ACR) measures. Superiority had not been uniformly shown in this regard at 6 months and it was this point that was intended to be conveyed in the media release. The Panel was concerned about the general claims for superiority. The media release also contained the claim ‘No previous biologic therapy has demonstrated superiority compared to MTX’ which was not so. The Panel noted that the media release had been sent to UK national and medical media. The product was not authorized in the UK and the media release was extremely positive; it used ‘novel’, ‘innovative’ and ‘most exciting’ to describe the product. The Panel considered that the media release was not factual and that the results of a clinical study had not been presented in a balanced way. The media release would raise unfounded hopes of successful treatment. Thus the Panel ruled a breach of the Code.
The Panel considered that given its comments above high standards had not been maintained. A further breach of the Code was ruled.
Roche Products Ltd and Chugai Pharma UK Ltd made a joint voluntary admission about a media release (ref PRX3158) concerning tocilizumab issued on 13 June.
Claims
New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis’
and
‘This new data, presented today at the European League Against Rheumatism (EULAR) meeting in Paris, makes tocilizumab the first and only biologic therapy to have achieved superiority over MTX [methotrexate]’
The first claim was the headline to the media release and the second claim appeared within the media release.
COMPLAINT
The companies brought the Authority’s attention to a media release they had issued on 13 June 2008. This media release related to the presentation of new clinical data on tocilizumab, a biologic therapy currently under consideration for marketing authorization by the US and European regulatory authorities for the management of rheumatoid arthritis. These data were presented at the European League Against Rheumatism (EULAR) meeting in Paris.
Roche and Chugai stated that following discussions with Wyeth Pharmaceuticals it had become apparent that the claims at issue might be considered factually incorrect when read alone and therefore might be in breach of the Code, in particular Clause 7.
Inter-company dialogue had been ongoing, with a request by Wyeth for a corrective statement to be published in scientific journals. This was considered; however, as tocilizumab was currently unlicensed the issuing of such a statement had been deemed by Roche and Chugai to be unachievable without potentially being in breach of the Code. Therefore inter-company dialogue had been unsuccessful and thus Roche and Chugai had decided that a voluntary admission was the only appropriate course of action.
Paragraph 5.4 of the 2008 Constitution and Procedure provided that the Director should treat a voluntary admission as a complaint if it related to a potentially serious breach of the Code or if the company failed to take appropriate action to address the matter. Issuing a potentially misleading press release was a serious matter and the admission was accordingly treated as a complaint.
When writing to Roche and Chugai, the Authority asked them to respond in relation to Clauses 2, 9.1 and 20.2 of the 2006 Code which were the same in the 2008 Code though numbered differently Clause 20.2 being Clause 22.2 in the 2008 Code. This case was considered under the 2008 Constitution and Procedure.
RESPONSE
Roche and Chugai submitted a joint response and explained that tocilizumab was currently being reviewed by the EU and US regulators. Market authorization in the EU was anticipated in 2009.
Tocilizumab was the first anti-interleukin 6 (IL-6) receptor antagonist to be developed.
The media statement for tocilizumab ‘New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis’ was issued on 13 June following the presentation of new data at the EULAR annual meeting in Paris. This media release was adapted from the global press release and was issued from the UK to the UK national and medical media. It was signed off in accordance with the approval and certification and public relations standard operating procedures (SOPs) of both Roche and Chugai.
The media release covered two phase III trials. The claims at issue related to the presentation of a phase III trial on the use of tocilizumab monotherapy compared with MTX monotherapy in patients with active rheumatoid arthritis who had not been treated with MTX within 6 months prior to randomization, the AMBITION trial (TocilizumAb versus Methotrexate double-Blind Investigative Trial In mONtherapy).
Patients were randomized in the 24 weeks, doubleblind, double-dummy parallel group, phase III study to either 8mg/kg tocilizumab every 4 weeks or to an escalating MTX dose of 7.5-20mg weekly. The primary analysis for non-inferiority used the per protocol population (n=524), and the secondary analysis for superiority used the intention to treat (ITT) population (n=570). The demonstration of superiority was based on the regulatory authority required efficacy measures of the ACR20, 50 and 70 scores. The American College of Rheumatology (ACR) scoring system was a composite measure and represented percentage improvement from baseline at defined time points. Because rheumatoid arthritis was a chronic systemic disease, that was probably best described as a syndrome, efficacy needed to be assessed beyond just the improvement in a patient’s joints or inflammatory markers and must account for both the physical and psychological effects of the disease. As such the ACR scoring system was made up of the following parameters, tender joint count, swollen joint count, patient’s assessment of pain, patient’s and physician’s global assessments of disease activity, patient’s assessment of physical function, and laboratory evaluation of one acutephase reactant eg C-reactive protein.
In defining a patient’s ACR20 improvement following the initiation of treatment a 20% improvement in tender and swollen joint counts and 20% improvement in 3 of the 5 remaining ACR core set measures (patient and physician global assessments, pain, disability, and an acute-phase reactant) was needed. For an ACR50 a 50% improvement would be needed and so forth.
The ACR measures sampled the broad range of improvement in rheumatoid arthritis, and all were at least moderately sensitive to change. Many of them predicted other important long-term outcomes, including physical disability, radiographic damage, and death.
When looking at the results of the AMBITION study the mean baseline characteristics were similar between groups. Non-inferiority was demonstrated for the primary endpoint ACR20 response at week 24 (71% tocilizumab/52% MTX). This led on to show that tocilizumab was superior to MTX treatment, with a higher proportion of ACR20/50/70 responders at week 24 (table below).
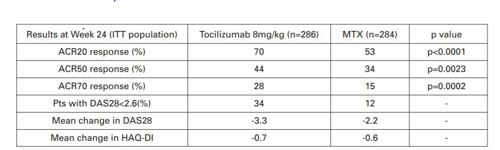
To further support the superiority of tocilizumab over MTX a higher proportion of patients achieved a good/moderate EULAR response as early as week 2 (64% tocilizumab/19% MTX), with rates reaching 82% vs 65%, respectively, at week 24.
These results were very significant as this was the first time any biologic therapy had demonstrated superiority across all ACR measures as well as DAS remission rates. This was achieved at 6 months.These results formed the basis of the media release and the two claims at issue.
The relevant section of the media statement stated:
‘New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis
Two new international studies also show high remission rates in patients treated with this novel therapy Welwyn Garden City 13 June 2008: The innovative rheumatoid arthritis drug tocilizumab has shown superiority over current standard of care, methotrexate (MTX), by achieving a greater reduction of signs and symptoms at 6 months in patients suffering from rheumatoid arthritis (RA).
This new data, presented today the European League Against Rheumatism (EULAR) meeting in Paris, makes tocilizumab the first and only biologic therapy to have achieved superiority over MTX.’
At this point of sign off Roche and Chugai believed that the context in which these claims were made was sufficiently clear to allow a distinction to be made between what was seen in the AMBITION trial compared with what had been shown with other biologic agents in similar populations. However Roche and Chugai had now raised this matter with the Authority as it recognised that the claims, when read alone, could potentially misrepresent the overall evidence base. Roche and Chugai also recognised that superiority of one therapy over another could be demonstrated in many different ways and therefore careful explanation for the basis of such a claim was needed.
Roche and Chugai noted when approving the media release that, on systematic review other biologic therapies had shown superiority, but either using other patient assessments, such as the DAS28 score or only part of the ACR scoring system eg ACR20 and 50 but not 70. Alternatively X-ray changes had been reported. However, superiority had not been uniformly shown at 6 months as with tocilizumab, and it was this point that was intended to be conveyed in the media release.
Two other studies reported the efficacy and safety of biologic monotherapy vs MTX monotherapy in the management of early rheumatoid arthritis.
Bathon et al (2000) compared etanercept (ETN) and MTX in patients with early rheumatoid arthritis. This study’s primary end point looked at the ACR-N of ETN 10mg twice weekly subcutaneous (sc), ETN 25mg twice weekly sc (licensed dose) and MTX. The ACR-N gave the overall response of each patient by calculating the smallest degree of improvement from baseline in the number of tender joints, the number of swollen joints and the median of the five remaining ACR criteria described above. Therefore the ACR-N represented the cumulative effect over time. When the ACR20, 50 and 70 were observed the ETN 25mg group showed significant improvement over MTX at six months for ACR70 only (p<0.05), the ACR20 and 50 were non significant. Significance at 4 months was shown at ACR20, 50 and 70. At no point beyond 6 months was there a significant difference between groups for ACR20, 50 and 70.
The ACR-N over 6 months was significant over time demonstrating rapid improvement in the patient’s condition but this measure alone could not demonstrate that ETN was superior to MTX at 6 months. This study also looked at radiographic changes over time. Radiographic measures were used to determine the disease modifying effect of one treatment against another. Bathon et al showed significant improvement in the ETN group over the MTX group at 6 months in two of the three scoring criteria, ie erosion and total Sharp score (p=0.001).
Joint-space-narrowing score however was non significant.
Genovese et al (2002) compared ETN and MTX in early rheumatoid patients and looked at radiographic changes at two years as a primary endpoint. ACR20, 50 and 70 were also recorded as a secondary endpoint. Statistical significance between the 25mg licensed dose and MTX for ACR20, 50 and 70 at 6 months was not formally reported. ACR20 at 24 months was significant between 25mg ETN and MTX groups, ACR50 and 70 were however non significant. Reviewing the radiographic endpoints significant improvement in the 25mg ETN group over MTX was seen at 24 months but no other time points were reported.
Other endpoints within the study also showed significance over MTX at 24 months including the Health Assessment Questionnaire that measured improvement in function and disability. The authors concluded that ‘the benefits of 25mg etanercept as monotherapy were shown to be superior to those of MTX at 2 years’.
When these data sets were compared with the tocilizumab trial results it could be seen that in terms of showing superiority there might be multiple differing opinions on what constituted clinical superiority. Roche and Chugai considered that as tocilizumab had demonstrated superiority across the entire ACR core set at 6 months, which was the clinical utility measure and time point employed by both the EU and US regulatory authorities in evaluating treatments for rheumatoid arthritis, they had the evidence to make the claims at issue. Roche and Chugai accepted that other therapies demonstrated superiority in some respect of the data and this should have been made clearer.
In considering Clause 20.2 (2006 Code) Roche and Chugai contested that the media release would bring unfounded hopes of successful treatment as tocilizumab was the first therapy to demonstrate superiority across the ACR core criteria which had not been achieved before. Within the media statement the safety profile of tocilizumab was clearly described. However, whilst the media release was factual, Roche and Chugai accepted that the superiority claims should be placed more clearly into context. However, although they accepted that the media release might have been better constructed, they strongly refuted that it had brought discredit to or reduced confidence in, the industry (Clause 2) or failed to maintain high standards (Clause 9.1).
This media release was legitimately issued as the information released at the EULAR meeting represented a significant development in the management of rheumatoid arthritis and was thus newsworthy. There was a high level of interest in terms of finding new treatments in this area as there was a significant unmet need. The media release reflected the specific results of the two trials within it in an accurate and objective manner. It was released in line with Roche and Chugai internal SOPs. The media release did not constitute promotion and was reviewed and signed off in good faith and with competent care. Roche and Chugai considered that high standards had been maintained throughout. They accepted that it needed to be clearer regarding the superiority claim; however in the companies’ opinion the media release did not represent the profile of tocilizumab in an unbalanced fashion compared with existing therapies.
Roche and Chugai therefore accepted that the media release might breach Clause 20.2 of the 2006 Code but refuted strongly that the material was in breach of Clauses 2 or 9.1.
PANEL RULING
The Panel considered that the heading to the media release, ‘New data reveals tocilizumab is the first and only biologic drug to show superiority over current standard of care in rheumatoid arthritis’ was a strong unqualified claim. The first paragraph of the media release explained that the current standard of care was methotrexate. The Panel noted the companies’ submission that other biologic therapies had shown superiority but unlike tocilizumab not across all ACR measures. Superiority had not been uniformly shown in this regard at 6 months and it was this point that was intended to be conveyed in the media release.
The Panel was concerned about the general claims for superiority. The media release also contained the claim ‘No previous biologic therapy has demonstrated superiority compared to MTX’ which was not so. The Panel noted that the media release had been sent to UK national and medical media. The product was not authorized in the UK and the media release was extremely positive; it used ‘novel’, ‘innovative’ and ‘most exciting’ to describe the product. The Panel considered that the media release was not factual and that the results of the AMBITION study had not been presented in a balanced way. The media release would raise unfounded hopes of successful treatment. Thus the Panel ruled a breach of Clause 20.2 of the 2006 Code.
The Panel considered that given its comments above high standards had not been maintained. A breach of Clause 9.1 was ruled.
Although noting its rulings above, the Panel did not consider that the media release warranted a ruling of a breach of Clause 2 which was used as a sign of particular censure and reserved for such use.
Proceedings commenced 7 August 2008
Cases completed AUTH/2154/8/08 7 October 2008
AUTH/2155/8/08 9 October 2008

