CASE AUTH/1841/5/06 TAKEDA v DAIICHI-SANKYO
Promotion of Olmetec
Takeda complained that a comparison of its product Amias (candesartan) with Olmetec (olmesartan) by Daiichi-Sankyo was unfair. The comparison was based upon Brunner et al 2003 which had compared olmesartan 20mg with candesartan 8mg in patients with hypertension. Both medicines currently had three doses within their usual dosing regimen for hypertension; patients were titrated according to response. Patients started on candesartan 8mg or olmesartan 10mg and would remain (be maintained) on those doses unless their blood pressure was not adequately controlled, at which time the dose of either might be doubled. If additional blood pressure reduction was required then the doses might be doubled again to candesartan 32mg or olmesartan 40mg. Brunner et al had compared the ‘usual maintenance dose’ of candesartan with the ‘optimal’ dose of olmesartan which was misleading. When the study was designed the starting dose for candesartan was only 4mg; the authors’ statement that the approved dose range for candesartan was 4, 8 and 16mg was out of date and inconsistent with the current summary of product characteristics (SPC) for Amias.
Takeda considered that the most appropriate comparison of the two products was candesartan 16mg vs olmesartan 20mg. Supporting data was provided including a meta-analysis of the dose response data for candesartan which showed that the ‘optimal’ dose for lowering blood pressure was 16mg and that the incremental benefit of moving from candesartan 8mg (starting and usual maintenance dose) to the optimal dose of 16mg was 2/2mmHg (Elmfeldt et al, 1997). A meta-analysis of the dose response data for olmesartan (Püchler et al, 2001) showed that the incremental benefit achieved by moving from the starting dose of 10mg to the ‘optimal’ dose of 20mg was 2.42/1.77mmHg. Takeda noted that in the US, the starting dose for olmesartan was 20mg (maximum dose of 40mg) and the starting dose for candesartan was 16mg (maximum dose of 32mg). This further supported Takeda’s stance that the most appropriate comparison would be between olmesartan 20mg and candesartan 16mg.
Takeda complained about a leavepiece and a journal advertisement which featured the allegedly unfair comparison. Takeda also complained about the promotional use of reprints of Brunner et al.
The Panel noted that Brunner et al stated that for candesartan, the approved dosage range was 4mg once daily as the starting dose, 8mg once daily as the usual maintenance dose and 16mg once daily as the maximum dose. This information was outdated. Since the paper was written the dose of Amias in hypertension had been revised upwards. The recommended initial dose and usual maintenance dose was now 8mg once daily which could be increased to 16mg once daily and thereafter further increased to a maximum of 32mg once daily if necessary. The SPC stated that the average additional effect of a dose increase from 16mg to 32mg once daily was small but that due to inter-individual variability a more than average effect could be expected in some patients.
The Olmetec SPC stated that the recommended starting dose was 10mg once daily. In patients inadequately controlled this dose could be increased to the optimal dose of 20mg. If patients remained inadequately controlled the dose could be increased to a maximum of 40mg daily. It was thus clear from the SPC that some patients would be controlled on Olmetec 10mg although the Panel had no way of knowing what percentage that might be.
The Panel considered that it was unfortunate that the Amias SPC and the Olmetec SPC used different terms to describe various doses. The Panel did not accept that ‘optimal dose’ and ‘usual maintenance dose’ necessarily meant one and the same thing as submitted by Daiichi-Sankyo.
The Panel noted that although Brunner et al had originally compared the midpoint doses of both candesartan and olmesartan, due to the upward revision in the candesartan dosing it now meant that the lowest dose of candesartan had been compared with the middle dose of Olmetec.
The leavepiece included a bar chart depicting the mean change in daytime blood pressure following once daily treatment with Olmetec 20mg and candesartan 8mg. The bar chart, however, did not state that the dose for Olmetec was the optimal dose whilst the candesartan dose was the starting and usual maintenance dose. It was thus difficult for readers to fully understand the clinical significance of the results. The Panel considered that in this regard the comparison in the leavepiece was misleading. Breaches of the Code were ruled. This ruling was appealed.
The advertisement featured the claim ‘Olmetec 20mg delivers more potent BP reduction than… candesartan 8mg’. A footnote stated that the medicines had been compared at their usual maintenance dose. This was not so. The dose for Olmetec was the optimal dose and the candesartan dose was the starting dose and usual maintenance dose. The Panel noted its comments above regarding the leavepiece. Further breaches of the Code were ruled. This ruling was appealed.
The Panel noted that the promotional use of an unsolicited reprint of an article about a medicine constituted promotion of that medicine and all relevant requirements of the Code must be observed. Brunner et al contained out of date information regarding the dose of candesartan. Unsolicited use of that paper was therefore misleading with regard to candesartan. Breaches of the Code were ruled. This ruling was accepted by Daiichi-Sankyo.
Upon appeal by Daiichi-Sankyo, the Appeal Board noted the bar chart in the leavepiece which compared the response to Olmetec 20mg and candesartan 8mg did not state that the Olmetec dose was the optimal dose which according to the SPC was only for those patients not adequately controlled at the recommended starting dose of 10mg, whilst the candesartan dose was the recommended starting and usual maintenance dose. It was thus difficult for readers to fully understand the clinical significance of the results. The Appeal Board considered that in this regard the comparison was misleading. The Appeal Board upheld the Panel’s rulings of breaches of the Code.
The advertisement featured the claim ‘Olmetec 20mg delivers more potent BP reduction than… candesartan 8mg’. A footnote stated that the medicines had been compared at their usual maintenance dose. The Appeal Board noted the dose for Olmetec was the optimal dose and the candesartan dose was the starting dose and usual maintenance dose. The Appeal Board considered that in practice such doses would be considered comparable. In this particular instance the Appeal Board considered that the basis of the comparison was clear. The Appeal Board ruled no breach of the Code.
Takeda UK Limited complained about the promotion of Olmetec (olmesartan) by Sankyo Pharma UK Ltd (now Daiichi-Sankyo). The items at issue were a leavepiece (ref OLM 212.1), a journal advertisement (ref OLM359) and the promotional use of a reprint of Brunner et al (2003) by representatives. Takeda supplied Amias (candesartan).
COMPLAINT
Takeda considered that a study comparing olmesartan 20mg with candesartan 8mg in patients with hypertension (Brunner et al, 2003), was an unfair comparison of the two products. Takeda alleged that use of data from this study in promotional materials was in breach of Clauses 7.2 and 7.3 because:
– Candesartan and olmesartan currently had three doses within their usual dosing regimen for hypertension; patients moved through the dose range for both according to blood pressure response.
o patients started on candesartan 8mg or olmesartan 10mg.
o patients would remain (be maintained) on candesartan 8mg or olmesartan 10mg unless their blood pressure was not adequately controlled, at which time the dose might be increased to candesartan 16mg or olmesartan 20mg.
o if additional blood pressure reduction was required then the dose might be increased to candesartan 32mg or olmesartan 40mg.
– The ‘usual maintenance dose’ of candesartan had been compared with the ‘optimal’ dose of olmesartan. Takeda alleged that this comparison was misleading.
– Brunner et al was designed prior to the starting dose for candesartan increasing from 4mg to 8mg. The publication stated that the approved dose range for candesartan was 4, 8 and 16mg which was now out of date and inconsistent with the current summary of product characteristics (SPC) for Amias.
– During intercompany discussions, and also in a previous case (Case AUTH/1523/10/03), DaiichiSankyo had stated its belief that the word ‘optimal’ (relating to the 20mg dose of olmesartan) was interchangeable with and had the same meaning as ‘maintenance’. Takeda disagreed and could not find any evidence to support DaiichiSankyo’s assumption. The meaning/definition of optimal in the Oxford dictionary was ’best or most favourable’; ‘most conducive to a favourable outcome’, whereas ‘maintenance’ was to ‘preserve’ or ‘keep up’.
Takeda considered that the most appropriate comparison of the two products was candesartan 16mg vs olmesartan 20mg.
– A published meta-analysis of the dose response data for candesartan showed that the ‘optimal’ dose for lowering blood pressure was 16mg and that the incremental benefit of moving from candesartan 8mg (starting and usual maintenance dose) to the optimal dose of 16mg was 2/2mmHg (Elmfeldt et al, 1997). Further titration from 16mg to the maximum dose of 32mg might provide additional benefit in some patients (Section 5.1 (Hypertension) Amias SPC).
– A published meta-analysis of the dose response data for olmesartan (Püchler et al, 2001) showed that the incremental benefit achieved by moving from the starting dose of 10mg to the ‘optimal’ dose of 20mg was 2.42/1.77mmHg.
– Takeda noted that in the US, the starting dose for olmesartan was 20mg (maximum dose of 40mg) and the starting dose for candesartan was 16mg (maximum dose of 32mg). This further supported Takeda’s stance that the most appropriate comparison would be between olmesartan 20mg and candesartan 16mg. The US dosing schedule had previously been used by Daiichi-Sankyo to support its case regarding the head-to-head comparison with losartan, valsartan and irbesartan (Case AUTH/1523/10/03).
Associated with the above, Takeda believed that the use of the Brunner et al as a promotional item (eg reprints provided by sales representatives) was also in breach of Clauses 7.2 and 7.3. In the discussion section of the paper, it clearly stated that the approved dosage range for candesartan was 4mg once daily as the starting dose, 8mg once daily as the usual maintenance dose and 16mg once daily as the maximum dose. This information was inaccurate, misleading and not consistent with the Amias SPC for candesartan in the UK.
Since Takeda’s intercompany discussions with Daiichi-Sankyo the journal advertisement had been published. This also contained the comparison at issue above in the claim ‘Olmetec potency puts you in control. Olmetec 20mg delivers more potent BP reduction than losartan 50mg, valsartan 80mg, irbesartan 150mg and candesartan 8mg. That’s the power you could prescribe’.
Takeda maintained that promotion using data from Brunner et al was inaccurate, misleading and not consistent with the SPC. These claims were in breach of Clauses 7.2 and 7.3 and hence these items (and any others that included this data) should be withdrawn from use.
RESPONSE
Daiichi-Sankyo firmly believed that Brunner et al was a fair and just comparison of the recognised maintenance doses of olmesartan (20mg) and candesartan (8mg) in the UK. The company therefore denied that the use of this data was in breach of Clauses 7.2 and 7.3.
Brunner et al was carried out in 2002 across 44 European centres and involved 643 patients. The study was conducted before Olmetec was launched in Europe and formed part of the regulatory submission. When the study was conducted the recognised doses of candesartan and the proposed dosing schedule of olmesartan for Europe were:
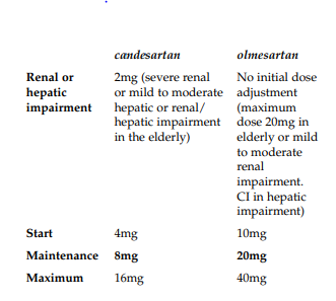
In effect four dose titrations (2mg, 4mg, 8mg and 16mg) existed for candesartan and three dose titrations (10mg, 20mg and 40mg) were proposed for olmesartan.
In March 2002 Olmetec was first approved in the EU in Germany via the Mutual Recognition Procedure with the three dose titration schedule ie 10mg as the start dose, 20mg as the maintenance dose and 40mg as the maximum dose. Market authorization in the UK was issued in May 2003. The SPC described the 20mg maintenance dose of olmesartan as ‘optimal’. As far as Daiichi-Sankyo was aware this term only appeared in the Olmetec SPC and not in the SPCs for other medicines in the same pharmacological class.
The dosing schedule for olmesartan had thus remained since launch as outlined above as 10, 20 and 40mg.
Following its EU launch in March 2002, and as part of the European promotional strategy for Olmetec, Brunner et al was used to support a comparison of the recognised maintenance doses for olmesartan (20mg) and for candesartan (8mg).
The data was first made available by Brunner in 2003 and then in the publication that followed later in 2003. More recently it had been made available in Brunner and Arakawa (2006). Hence, the data had been available in the scientific literature for over four years in a number of publications as well as being a secondary reference in other publications.
Daiichi-Sankyo believed that Takeda changed the starting dose of Amias from 4mg to 8mg in March 2003. A further change occurred in September 2004 when a 32mg maximum dose was introduced. However most notably the maintenance dose had remained as 8mg since the launch of the product in 1998.
Candesartan thus currently had five doses within the usual dose regimen whereas olmesartan had three.
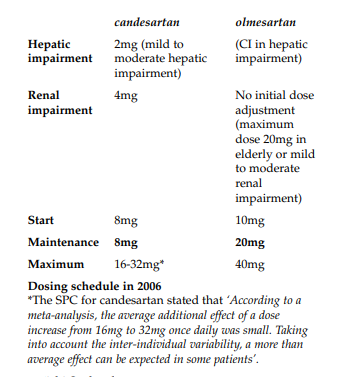
Daiichi-Sankyo believed that the dose changes that had occurred with candesartan had not changed the validity of the study comparison as the maintenance doses for the two products had remained the same since launch. Currently, it remained fair, accurate and was not misleading and was thus not in breach of Clauses 7.2 and 7.3.
In the arguments presented by Takeda to support the opinion that olmesartan 20mg should be compared with candesartan 8mg it considered two metaanalyses. For candesartan (Elmfedt et al) and for olmesartan (Püchler et al). Although these might appear similar in design there were some obvious differences which might make such a comparison invalid. The key difference between the two analyses were:
- The olmesartan analysis was twice the size of the candesartan analyses and thus the conclusions from the candesartan analyses were likely to be less robust
- The range of hypertension considered in the two analyses differed and groups were not comparable. Patients in the olmesartan group were more difficult to treat and had more severe hypertension (100-120mmHg) compared to (95114mmHg) in the candesartan group.
- The studies used in the candesartan analysis lasted
4-12 weeks whereas some studies included in the olmesartan analyses lasted 52 weeks (minimum 6 weeks). Longer term control was harder to maintain and this would influence the results analysed.
- The candesartan analysis was of a fixed dose nature with a defined group of patients receiving each therapy. The olmesartan analysis included some patients who were titrated to higher doses and thus were counted in more than one group.
- Normalisation rate analyses were not conducted in the candesartan analysis
- Responder rate and normalisation rate were not analysed as primary objective measures in the candesartan analysis
Daiichi-Sankyo did not agree with the scientific credibility of comparing the results of individually conducted analyses for different products with each other, particularly where there were differences in study size, patient type, study length and degree of hypertension being analysed. It was of the opinion therefore that this argument was weak.
In addition the conclusion drawn by Takeda was supported by selectively picking data just to show the incremental benefit of increasing the dose of olmesartan from 10 to 20mg being similar to that of moving from candesartan 8 to 16mg. As a consequence Daiichi-Sankyo believed Takeda’s conclusion was flawed since it did not consider the comparative placebo corrected BP response at the specific doses mentioned or across the remainder of the dose profile. If it were scientifically valid to draw conclusions from and compare results of two individually conducted meta-analyses then in DaiichiSankyo’s view this could only be done by looking at the entire dose range and the placebo corrected responses.
If one considered the placebo corrected diastolic blood pressure (DBP) and systolic blood pressure (SBP) reductions across the dose ranges reported ie 10-40mg olmesartan and 4-16mg candesartan there was very little difference in the comparative BP reductions as reported by Elmfedt et al and Püchler et al.
The placebo corrected reductions in DBP and SBP for olmesartan 20mg and candesartan 8mg were reported as being the same in both individually conducted analyses. This argument supported a fair maintenance dose comparison at 20mg and 8mg of olmesartan with candesartan.
Takeda stated that up-titrating candesartan from 16mg to 32mg might provide ‘some additional benefit’. This was also stated in the SPC. Takeda then suggested that this supported the supposition that by moving from 20mg to 40mg of olmesartan this would be the most appropriate comparison. Daiichi-Sankyo disagreed strongly with this opinion. If one considered the effect of upwards titration from 20mg to 40mg with olmesartan then the 40mg dose was statistically superior in terms of SBP lowering compared to 20mg (p=0.002), removal of the placebo effect provided similarly significant results (p=0.04) in favour of olmesartan 40mg for SBP reduction alone. Furthermore, although the statistical significance was not analysed, the 40mg dose also provided higher SBP normalisation rates (SBP≤130mmHg) 49% vs 45%, and (SBP≤135mmHg) 28% vs 20%.
When one considered DBP the greatest mean decrease from baseline in sitting DBP was observed for patients on the 40mg dose, 15.7mmHg compared with 7.6mmHg for patients on placebo. Again, although the statistical significance was not analysed, the 40mg dose also provided a higher DBP responder rate (DBP≤90mmHg or DBP decrease ≥10mmHg) 81% compared to 70% and higher normalisation rates (DBP≤90mmHg) 62% vs 51%, (DBP≤85mmHg) 31% vs 28%.
It was clear therefore that there was a difference that was both significant and measurable between the olmesartan 20mg and 40mg dose following up-titration. This differed from the small additional benefit seen when titrating up from 16mg candesartan to 32mg.
There was thus no clear scientific rationale in this respect to make a comparison as suggested by Takeda of the 16mg and 32mg doses with 20mg and 40mg of olmesartan.
Further support was gained for this argument by comparing the 80mg dose of olmesartan with candesartan 32mg to look at the indicative response.
If the results were further extrapolated to include doses up to 32mg candesartan (Reif et al) then the 80mg dose of olmesartan (not licensed) was similar in response to candesartan 32mg. Clearly this would remain to be evaluated and supported by a head-tohead clinical study but this would indicate the most fair comparison if this approach were to be used.
Daiichi-Sankyo believed that on the balance of evidence it remained fair, accurate and was not misleading to compare olmesartan 20mg with candesartan 8mg and thus the use of this comparison in clinical data was not in breach of Clauses 7.2 and 7.3.
Daiichi-Sankyo acknowledged that the Olmetec SPC did not explicitly specify a maintenance dose of olmesartan and that this differed from other medicines in the same class. Further complexity occurred due to the wording of the SPC which stated that the dose of Olmetec 20mg was the ‘optimal’ dose.
However Daiichi-Sankyo continued to believe, as it had maintained in its promotional material that since the launch of Olmetec, and as proven in Case AUTH/1523/10/03, the recognised maintenance dose of olmesartan was 20mg. This had been recognised independently by competitors, within the NHS, and in the published scientific literature. Furthermore the WHO ATC Daily Defined Dose (DDD) classification which listed the recognised comparative doses of molecules within a therapy class stated that the usual recognised DDD of candesartan and olmesartan were 8mg and 20mg respectively. This supported the rationale that the comparison of olmesartan 20mg with candesartan 8mg was valid and appropriate and that the recognised maintenance dose of olmesartan was 20mg. Daiichi-Sankyo was not aware of any evidence which suggested that 10mg of olmesartan should be the maintenance dose as stated by Takeda or that the appropriate comparator for olmesartan 20mg should be candesartan 16mg.
Daiichi-Sankyo disagreed with Takeda’s assertion that the terms ‘optimal’ and ‘maintenance’ were not interchangeable. The optimal effect was the best or most favourable outcome the best outcome must be to ‘maintain or preserve’ in this instance a patient’s blood pressure to the desired level. This would mean avoiding a sub-optimal or supra-optimal response by using too high or too low a dose which could have adverse consequences.
Daiichi-Sankyo also noted that Elmfeldt et al used the terminology ‘optimal’ with reference to candesartan. Within this publication it was stated that 8mg was an optimal dose for candesartan within the usual maintenance dose range of 8-16mg. This further supported the rationale for an interchangeable use of the terminology.
In its correspondence with Takeda, Daiichi-Sankyo maintained the clinical data comparison of candesartan 8mg and olmesartan 20mg was a fair and just comparison and the provision of this reprint was also fair as it supported the efficacy of olmesartan and candesartan at UK maintenance doses. However Daiichi-Sankyo acknowledged that the provision of the reprint with a now out-of-date start dose of 4mg and maximum dose of 16mg for candesartan was potentially misleading; although it did not change the meaning or conclusions of the study as this was a comparison of recognised maintenance doses which had not changed. Daiichi-Sankyo had offered to label the page in question with the correct dose schedule for candesartan on reprints provided by its salesforce. This offer was declined due to the difference in opinion with regards to the validity of the comparison.
Daiichi-Sankyo did not believe that the use of Brunner et al to support a maintenance dose comparison was misleading or inaccurate and had tried to ensure consistency with the Amias SPC. As a consequence Daiichi-Sankyo not consider that there was a breach of Clause 7.2 or 7.3 in this regard.
In summary Daiichi-Sankyo submitted that the maintenance dose of olmesartan had remained as 20mg since launch of the product and this was well recognised.
The 8mg maintenance dose of candesartan had remained unchanged since its launch in 1998 and also remained well recognised. However, the candesartan dose schedule had changed at least twice since 1998 and it was now relatively complex with the availability of five possible doses dependent on patient type.
As a consequence Brunner et al remained a valid comparison of current recognised maintenance doses in the UK and its use and interpretation remained unaffected by the changes in dose titration scheme for candesartan that had occurred.
Daiichi-Sankyo therefore believed that it was not in breach of Clauses 7.2 and 7.3 as alleged.
PANEL RULING
The Panel noted that Brunner et al stated that for candesartan, the approved dosage range was 4mg once daily as the starting dose, 8mg once daily as the usual maintenance dose and 16mg once daily as the maximum dose. This information was out dated. Since the paper was written the dose of Amias in hypertension had been revised upwards. The SPC stated that the recommended initial dose and usual maintenance dose was 8mg once daily. The dose could be increased to 16mg once daily and if blood pressure was not sufficiently controlled after 4 weeks of treatment with 16mg once daily, the dose could be further increased to a maximum of 32mg once daily. The SPC stated that the average additional effect of a dose increase from 16mg to 32mg once daily was small but that due to inter-individual variability a more than average effect could be expected in some patients.
The Olmetec SPC stated that the recommended starting dose was 10mg once daily. In patients where blood pressure was inadequately controlled at this dose, the dose could be increased to the optimal dose of 20mg. If patients remained inadequately controlled the dose could be increased to a maximum of 40mg daily. It was thus clear from the SPC that some patients would be controlled on Olmetec 10mg although the Panel had no way of knowing what percentage that might be.
The Panel considered that it was unfortunate that the Amias SPC and the Olmetec SPC used different terms to describe various doses. The Panel did not accept that ‘optimal dose’ and ‘usual maintenance dose’ necessarily meant one and the same thing. In the Panel’s view the ‘usual maintenance dose’ of an antihypertensive was that dose which controlled most people’s blood pressure. In the Panel’s view the ‘optimal dose’ of a medicine encompassed consideration of its efficacy vs side effects and was the most favourable balance of the two but what was an optimal dose (and possibly also the usual maintenance dose) in one patient might be a suboptimal dose in another.
The Panel noted that although Brunner et al had originally compared the midpoint doses of both candesartan and olmesartan, due to the upward revision in the candesartan dosing it now meant that the recommended initial dose and usual maintenance (lowest) dose of candesartan had been compared with the optimal (middle) dose of Olmetec.
The leavepiece included a bar chart depicting the mean change in daytime blood pressure following once daily treatment with Olmetec 20mg and candesartan 8mg. The bar chart, however, did not state that the dose for Olmetec was the optimal dose whilst the candesartan dose was the starting and usual maintenance dose. It was thus difficult for readers to fully understand the clinical significance of the results. The Panel considered that in this regard the comparison in the leavepiece was misleading. Breaches of Clauses 7.2 and 7.3 were ruled. This ruling was appealed by Daiichi-Sankyo.
The advertisement featured the claim ‘Olmetec 20mg delivers more potent BP reduction than… candesartan 8mg’. A footnote stated that the medicines had been compared at their usual maintenance dose. This was not so. The dose for Olmetec was the optimal dose and the candesartan dose was the starting dose and usual maintenance dose. The Panel noted its comments above regarding the leavepiece. Further breaches of Clauses 7.2 and 7.3 were ruled. This ruling was appealed by Daiichi-Sankyo.
The Panel noted that the promotional use of an unsolicited reprint of an article about a medicine constituted promotion of that medicine and all relevant requirements of the Code must be observed. Brunner et al contained out of date information regarding the dose of candesartan. Unsolicited use of that paper was therefore misleading with regard to candesartan. Breaches of Clauses 7.2 and 7.3 were ruled. This ruling was accepted by Daiichi-Sankyo.
During its consideration of the advertisement the Panel noted that the supplementary information to Clause 7 stated that claims must be capable of standing alone; in general they should not be qualified by the use of footnotes and the like. The Panel requested that Daiichi-Sankyo be reminded of this advice.
APPEAL BY DAIICHI-SANKYO
Daiichi-Sankyo submitted that the bar chart in the leavepiece and claim in the advertisement were valid, stand alone statements. As a result, Daiichi-Sankyo did not accept the Panel’s ruling that the claims in these materials were misleading in breach of Clauses 7.2 and 7.3.
Daiichi-Sankyo stated that the leavepiece at issue was no longer in use.
Daiichi-Sankyo submitted that the following points formed the basis of its appeal:
- In Case AUTH/1523/10/03, the Panel held that a comparison of Olmetec 20mg was a fair comparison with the start and maintenance doses of valsartan, losartan and irbesartan (in each case where such start and maintenance doses were identical);
- The maintenance dose of candesartan had remained unchanged at 8mg since its launch in 1997. The entire dosage scheme of Olmetec
(including in particular the 20mg recognised ‘optimal’ dose) had not changed since its launch in 2003. Accordingly the scientific validity of the comparison in Brunner et al between the maintenance dose of candesartan (8mg) with the optimal dose of Olmetec (20mg) remained sound;
- It was not appropriate to take a semantic approach to the significance of the wording in SPCs in cases (such as with sartans) where there was inconsistent terminology. Four of the seven sartans did not specifically use the ‘maintenance dose’ terminology.
- Data on actual use and dosing trends should be taken into account. In this regard over the 36 months since its launch, Olmetec 20mg had become the most used dose of Olmetec in the UK (International Marketing Services (IMS) British Pharmaceutical Index (BPI) to June 2006), indicated by packs sold. The trend was also towards Olmetec 20mg being the most used dose of Olmetec in terms of patients being prescribed
any single dose. Furthermore, Olmetec 20mg had a higher persistence on therapy compared to Olmetec 10mg indicating that more patients remained on this dose once they were placed on it (IMS DIN-LINK data to May 2006).
- Well respected and authoritative published data including the WHO and Martindale (34th edition) recognised that Olmetec 20mg was the usual dose or maintenance dose.
Daiichi-Sankyo submitted that the Panel’s view that the term ‘optimal’ was not interchangeable with that of ‘maintenance’ seemed to contradict its ruling in Case AUTH/1523/10/03 where it was considered fair to compare Olmetec 20mg, as the ‘optimal’ dose, with the starting and maintenance dose of losartan 50mg, valsartan 80mg and irbesartan 150mg (Oparil et al).
Daiichi-Sankyo submitted that in Case AUTH/1523/10/03 the Panel appeared to accept its argument to the effect that the maintenance dose of Olmetec was 20mg and thus the comparison of these doses in the UK as maintenance doses was valid. In particular Daiichi-Sankyo noted the Panel’s comment that in relation to the treatment of hypertension the start and maintenance doses of each of the compared sartans considered were one and the same and the Panel did not consider that the claims at issue compared the titration dose [20mg] of Olmetec with the starting doses of losartan, valsartan and irbesartan as alleged. Therefore it must be concluded that the comparison was of recognised maintenance doses. As discussed below there had been no change in the maintenance dose of either Olmetec or candesartan during this period.
Daiichi-Sankyo was extremely concerned that the Panel’s ruling was in apparent contradiction of its 2003 ruling which substantially informed the company’s use of Brunner et al in the promotion of Olmetec since that time. Daiichi-Sankyo’s surprise and disappointment was heightened by the fact that Brunner et al had been used since the launch of Olmetec (and since the 2003 Panel ruling) without complaint despite the candesartan dosage changes of which the starting and maintenance amalgamation occurred in May 2003.
Daiichi-Sankyo noted that in the previous ruling in favour of Olmetec the middle dose was accepted as being a fair comparison to the lowest dose of the other products using the above rationale. This ruling appeared to show significant inconsistency in the ruling made by the Panel in the current case.
Daiichi-Sankyo noted that the Panel had noted that the dosing of candesartan had been revised upwards. Whilst the maximum dose had increased to 32mg (December 2004) and the start dose to had been revised to 8mg from 4mg (May 2003) the maintenance dose had remained as 8mg since the launch of the product in 1997. Furthermore the Olmetec dose had not changed since its launch with 10mg being the start dose, 20mg the quoted ‘optimal’ dose and 40mg the maximum dose. Since the maintenance doses in question had not changed during this period for either Olmetec or candesartan, Daiichi-Sankyo considered the comparison of these doses was justified.
Daiichi-Sankyo noted that Takeda had cited the ‘Oxford dictionary’ definitions of ‘maintenance’ and ‘optimal’. The Panel considered it to be ‘unfortunate’ that the candesartan SPC and Olmetec SPC used different terms. In the circumstances the Panel considered that it was entitled to ‘assume’ (without giving any reasoning therefore) that the ‘usual maintenance dose’ of an antihypertensive was one which controlled most people’s blood pressure. It further decided (again without giving any rationale) that the ‘optimal dose’ was a dose which ‘encompassed consideration of its efficacy vs side effects and was the most favourable balance of the two but what was an optimal dose (and possibly also the usual maintenance dose) in one patient might be sub-optimal in another’.
Daiichi-Sankyo noted that only three of the seven marketed sartans had a specifically defined maintenance dose. The SPCs for Micardis (telmisartan), Teveten (eprosartan), Diovan (valsartan), and Olmetec, did not specify a recognised maintenance dose. Instead terminology such as ‘usually effective dose’ and ‘recommended dose’ as well as Daiichi-Sankyo’s ‘optimal dose’ was used in relation to other sartans. It was generally accepted that such terms corresponded in the mind of clinicians to the term ‘maintenance dose’.
Daiichi-Sankyo further challenged the Panel’s ‘assumed’ definitions for ‘usual maintenance dose’ and ‘optimal dose’. The Panel defined ‘usual maintenance dose’ ‘as the dose which controlled most patients’ blood pressure’. Daiichi-Sankyo did not regard either of the terms, ‘usually effective dose’ (telmisartan) or ‘recommended dose’ (valsartan, eprosartan) to come within the Panel’s definition of the maintenance dose. A ‘usually effective dose’ was normally defined as that dose which was ‘commonly encountered, experienced, or observed providing an expected response’; whilst a recommended dose would be considered the ‘approved, favoured or endorsed dose’. Despite this, these doses were widely regarded as the individual maintenance doses of the products in question. In short, if Olmetec 20mg was not to be considered a valid comparator for candesartan 8mg for maintenance purposes on the apparently sole basis that ‘optimal’ and ‘maintenance’ were not synonymous then it would seem that telmisartan 40mg, eprosartan 600mg, and valsartan 80mg, would each face similar difficulties.
Daiichi-Sankyo submitted that the Panel’s definition of ‘optimal dose’ of the medicine as one which encompassed consideration of its efficacy vs side effects and was the most favourable balance of the two but what was an optimal dose (and possibly also the usual maintenance dose) in one patient might be sub-optimal in another must also be challenged. It was equally arguable that the dose required as the maintenance dose in one patient might be different to that required in another and thus might similarly be ‘sub-optimal’.
Daiichi-Sankyo submitted that with specific reference to the Panel’s definition of optimal dose referred to above it noted that in the regulatory process the determination of what was the optimal dose was made with reference to efficacy. With respect to the Panel’s view that side effects should form part of the criterion of the definition of optimal, the tolerability of sartans was not dose-related and this had been demonstrated with Olmetec (Smith 2002).
Daiichi-Sankyo agreed with the Panel and with Takeda that the maintenance dose of a product was that dose which controlled most patients’ blood pressure, however this could not be determined just by reference to the wording in the SPC (which was divergent) but by reference to actual clinical and use data and by other published data and information as a product’s usage became established.
Daiichi-Sankyo submitted that where SPC language was inconsistent (as with sartans), consideration must also be given to actual data during use as indicating a product’s most frequent actual maintenance dose. In this regard the period of time a product had been made available, its relative growth and the trend in dosing since launch, as prescribers became familiar with the product, must be considered as indicators.
Daiichi-Sankyo noted that the Panel had stated that some patients would be controlled on Olmetec 10mg but it had no way of knowing what percentage that might be. Daichi-Sankyo thus set out various sets of data which demonstrated that Olmetec 20mg was either the most used dose of Olmetec or was trending quite clearly towards this in the UK; comparable data for candesartan over the same time period since its launch was also provided.
Daiichi-Sankyo submitted that it was evident from the information provided that over the 3 years since its launch, the 20mg dose would now become the most used dose in the UK. In particular Daiichi-Sankyo noted that over the last six months and in particular at the time of the publication of the promotional item in question, the 20mg dose was either the most used, or had achieved equivalent sales levels and when one took into account the volume of new patients, (Olmetec was one of the fastest-growing sartans in the UK market (IMS BPI to June 2006)) this clearly indicated the predominant use of the 20mg dose for maintenance purposes.
Over the same time period in the candesartan life cycle ie 3 years since launch, the 8mg dose did not at any time surpass that of 4mg dose despite the fact that the SPC clearly stated that the 8mg dose was the ‘maintenance dose’.
Daiichi-Sankyo recognised that the data related to packs sold and did not directly indicate patients on a specific dose. However there was additional supportive data which reinforced its argument that Olmetec 20mg dose had in effect become the usual maintenance dose. In the 6-month period to June 2006, 46% of patients received Olmetec 20mg, compared with 42% who received Olmetec 10mg. The information provided showed the actual number of patients on a particular dose of Olmetec since launch in the UK. Again the trend 3 years into launch indicated a growing percentage of patients on Olmetec 20mg.
Daiichi-Sankyo submitted that although it did not have access to equivalent data for candesartan since launch in 1997 it had data from 2001 onwards, a full four years into launch. This demonstrated that even though candesartan was stated throughout to have a maintenance dose of 8mg, more patients still received the 4mg as a starting dose than the 8mg dose until 2004, some seven years after launch.
Daiichi-Sankyo submitted that it was of value to consider the persistence rate on treatment (the rate at which patients stayed on any one particular dose). It would be expected that the persistence rate for a maintenance dose would be higher than the persistence for the starting dose, as better control would be evident.
Daiichi-Sankyo submitted that Olmetec 20mg had a higher rate of persistence to therapy than Olmetec 10mg with more patients being maintained on treatment over a 12-month period following initiation on therapy. Reasons for this included the need for upwards titration of dose, lack of efficacy, change of treatment, and non-compliance. This further demonstrated that more patients were maintained on Olmetec 20mg following initiation.
Daiichi-Sankyo submitted that its position that Olmetec 20mg was the maintenance or usual dose of Olmetec was supported by: the World Health Organisation; Martindale; Brunner and Arakawa and promotional material for Micardis and Aprovel.
Finally Daiichi-Sankyo noted that the Panel referred extensively to Elmfedt et al and Püchler et al. Whilst there was no direct reference to the meta-analyses in question in the Panel’s ruling, in the event that it did inform to any extent the Appeal Board’s thinking on this matter Daiichi-Sankyo specifically repeated its arguments in response to the complaint in that it was generally accepted that this method of comparing individual meta-analyses conducted independently with differing patient populations was not scientifically valid. Daiichi-Sankyo reiterated points relevant to the appeal from its response to the complaint.
In conclusion Daiichi-Sankyo submitted that on the balance of evidence it was fair, accurate and not misleading to compare Olmetec 20mg with candesartan 8mg and thus the use of this comparison in clinical data was not in breach of Clauses 7.2 and 7.3. In addition, Daiichi-Sankyo was particularly concerned that the Panel’s ruling contradicted its 2003 ruling in relation to the dosing of Olmetec. DaiichiSankyo understood that the two cases might differ in certain respects but there had been no change to the specific doses in question and it was not unreasonable to expect the Panel to be consistent in its rulings on such matters.
COMMENTS FROM TAKEDA
Takeda noted that the sartans involved in Case AUTH/1523/10/03 (losartan, irbesartan and valsartan) had only two doses within their usual treatment range (excluding special populations where tolerability considerations were of particular importance, such as those with renal or hepatic impairment) unlike olmesartan and candesartan which had three. These other sartans had a single combined starting and maintenance dose and a maximum dose. There was not a third (middle) dose that allowed further titration and optimisation of their maintenance dose. For this reason, the issues involved in the present case, Case AUTH/1841/5/06, were different to those in Case AUTH/1523/10/03 which rendered it invalid as a suitable case precedent.
Takeda noted that when Brunner et al was designed and conducted, candesartan had three doses within its usual range for hypertension – 4mg starting dose, 8mg maintenance dose and 16mg maximum dose. In 2003 this changed to two doses with the removal of the 4mg as the starting dose. The change to the dosing schedule of candesartan was completed in December 2004 when the maximum dose was increased to 32mg, thereby shifting the whole dosing schedule upwards. Therefore, the dosing regimen that was applicable when Brunner et al was designed in the early 2000s was not appropriate today.
Takeda also noted that the treatment regimens used in Brunner et al (8 week duration) were not consistent with recognised and current medical practice or the current SPCs for either olmesartan or candesartan. In line with the UK SPC for candesartan, patients should commence treatment on 8mg and after 4 weeks should have their blood pressure monitored and the dose increased to 16mg if necessary (most of the antihypertensive effect of an individual dose was achieved after 4 weeks). In line with the SPC for olmesartan, patients should commence treatment on 10mg before up titrating to 20mg (the maximal effect was seen after 8 weeks). Patients in Brunner et al did not have the option of up-titration to candesartan 16mg after 4 weeks.
Takeda noted that hypertension was a chronic condition and treatment was long-term and usually lifelong. Patients were treated according to their response to a medicine and subsequent reduction in blood pressure. A patient would be titrated on a particular treatment until they achieved their target blood pressure. The dose of a medicine that enabled a patient to achieve target was used to maintain that patient and became their ‘maintenance’ dose. Patients usually commenced treatment with candesartan 8mg and if sufficient BP lowering was achieved they stayed and were ‘maintained’ on this dose. Patients whose blood pressure was not sufficiently lowered with 8mg had their dose increased in line with the SPC to 16mg and if sufficient BP reduction was achieved they were maintained on this dose. For some patients, additional benefit might be gained by increasing the dose further to 32mg, or alternatively adding in a different class of antihypertensive in line with NICE recommendations. When the dosing range for candesartan shifted upwards, the 16mg dose changed from being the maximum dose to becoming a ‘maintenance’ dose and based on the dose response data was clearly the optimal maintenance dose for candesartan.
Takeda stated that it was unfortunate that wording used by regulatory authorities could sometimes be ambiguous and inconsistent. This was particularly so when the inconsistencies occurred within a single class of medicines. What was consistent, however (and not dependent on the nuances of language), was the patient path for each medicine.
Candesartan and olmesartan had three doses within their usual dosing regimen for hypertension; patients moved through the dose range for both according to blood pressure response:
- Patients started on candesartan 8mg or olmesartan 10mg
- Patients remained (maintained) on candesartan 8mg or olmesartan 10mg unless their blood pressure was not adequately controlled, at which time the dose might be increased to candesartan 16mg or olmesartan 20mg. The SPCs for both products clearly stated that the dose was increased only if the patient required additional blood pressure lowering. If the patient’s BP was lowered sufficiently on these doses (candesartan 16mg, olmesartan 20mg) then they would remain (be maintained) on these doses.
- If further blood pressure reduction was required then the dose might be increased to the maximum doses of candesartan (32mg) and olmesartan (40mg).
Takeda alleged that based on the dose response metaanalyses previously submitted for each of the products (Elmfeldt et al and Püchler et al), it was clear that the optimal dose for each product was 16mg (candesartan) and 20mg (olmesartan).
Takeda noted the timing of the licensing of these two medicines. Candesartan was one of the first sartans to be launched in 1997. At this time, the sartans were a new class of medicines with little long-term safety data and therefore, the dosing regimens tended to be on the conservative (low) side. Olmesartan was launched in 2003 (6 years later) when there was significant safety data and greater confidence in the class along with data from several large outcome studies. Takeda (in collaboration with AstraZeneca) had aimed to address this by submitting variations to the regulatory authorities to increase and shift the dosing range of candesartan upwards from that originally approved in 1997.
Takeda noted that data and claims used within promotional material should be based on robust scientific data and not sales data which was not statistically valid and subject to commercial influences.
Takeda alleged that all the usage data presented by Daiichi-Sankyo had to be viewed with consideration of the following potential biases:
- It was not appropriate to compare the launch/uptake dynamics of a product launched into a brand new class and one launched 6 years later when the class had matured and there was more safety data available and confidence in the class (ie 1997 vs 2003).
- The uptake of various strengths of a product would be significantly influenced by many factors including the level of promotion around each strength, pricing and available discount schemes. It should be noted that the promotion for Olmetec in the UK was heavily focussed on the 20mg dose.
- The fact that there was such a difference in dosage use across Europe (vs UK) for olmesartan further supported the influence that outside factors other than scientific data might have.
- It was not clear whether some of Daiichi-Sankyo’s data took account of the different pack sizes of 4mg candesartan (available in 7s and 28s). If it did not then it would be biased towards the 4mg strength. The most appropriate unit would be ‘28 day equivalents’.
Takeda noted that in the data presented by DaiichiSankyo, it could clearly see that over time (and consistent with increased confidence and comfort with the sartan class and changes to the dose range of candesartan) the use of the higher strengths of candesartan had increased. This was particularly noticeable for the 16mg dose, which overtook the 4mg strength in 2004 and was clearly catching up with the 8mg dose. Takeda also noted that since May 2004 it had not had a traditional sales force promoting candesartan and this shift in use was therefore not biased by promotional activity. During 2005, Takeda’s share of the sartan market was 2.4% (share of calls and share of total promotional spend; IMS MPI Overview, MAT Dec 2005). The equivalent share for Olmetec was: 12.4% and 13.7% for calls and total promotional spend respectively (IMS MPI Overview, MAT Dec 2005).
Takeda alleged that it also appeared that in its response, Daiichi-Sankyo had misinterpreted the data presented which clearly showed that most patients received the 8mg dose of candesartan. This was consistent throughout 2001-2006. It was the 4mg and 16mg strengths that crossed over in 2004 (as discussed above).
Takeda noted as discussed previously, hypertension was a chronic disease and all doses could be ‘maintenance’ doses. Different patients required different doses to maintain their blood pressure at an acceptable level. Both 8mg and 16mg of candesartan were maintenance doses. Based on its dose response data (Elmfeldt et al), Takeda alleged that candesartan 16mg was its optimal maintenance dose (i.e. the most efficacious dose for lowering BP). For olmesartan, both 10mg and 20mg were ‘maintenance’ doses with the appropriateness of either dose being determined by patient response. Olmesartan 20mg was viewed to be the optimal maintenance dose of olmesartan.
Takeda noted that Daiichi-Sankyo had referred to the World Health Organisation (WHO) daily defined dose (DDD). Takeda noted from the WHO website that the DDD was a unit of measurement and did not necessarily reflect the recommended or prescribed daily dose. The DDD was not designed to necessarily reflect therapeutically equivalent doses and it was acknowledged that the average daily dose might change over time. The DDD was designed solely to maintain a stable system of medicine consumption measurement which could be used to follow trends in utilization of medicines within and across therapeutic groups. The WHO specifically stated that the recommendation of a substance in the ATC/DDD system was not a recommendation for use, nor did it imply any judgements about efficacy or relative efficacy of medicines and groups of medicines. The DDD for candesartan was allocated at the time of launch within the EU (1997) at which time the starting dose was 4mg, maintenance dose was 8mg and maximum dose was 16mg. WHO requested that any changes to the DDD were kept to a minimum and avoided as far as possible. Too many alterations would always be disadvantageous for long-term studies on medicine utilization.
Takeda considered that the most appropriate comparison would be between candesartan 16mg and olmesartan 20mg (ie their optimal maintenance doses). With the upward shift of the whole dosing range for candesartan since Brunner et al was designed and conducted, what might have been a fair comparison then (early 2000s) was no longer appropriate and valid. The appropriateness of candesartan 16mg vs olmesartan 20mg being the most fair and scientifically valid comparison was further supported by the approved dosing in the US where the starting dose of candesartan was 16mg with 32mg as the maximum dose and for olmesartan, 20mg was the starting dose with 40mg being the maximum.
In conclusion, Takeda considered that the comparison of olmesartan 20mg with candesartan 8mg was not fair and was misleading, in breach of Clauses 7.2 and 7.3.
APPEAL BOARD RULING
The Appeal Board examined the case report for the previous case, Case AUTH/1523/10/03, referred to by Daiichi-Sankyo. The Panel’s ruling had not been appealed and the complaint was from Novartis not Takeda. The case considered in 2003 was distinguishable in that it had been considered before the change of the starting dose for candesartan from 4mg to 8mg and the introduction of the 32mg dose. These changes were completed in December 2004. Each case under the Code had to be considered on its own particular merits.
The Appeal Board noted the bar chart in the leavepiece depicted the mean change in daytime blood pressure following once daily treatment with Olmetec 20mg and candesartan 8mg. There was no statement, however, as to what these doses were ie that the dose for Olmetec was the optimal dose which according to the SPC was only for those patients not adequately controlled at the recommended starting dose of 10mg, whilst the candesartan dose was the recommended starting and usual maintenance dose. It was thus difficult for readers to fully understand the clinical significance of the results. The Appeal Board considered that in this regard the comparison in the leavepiece was misleading. The Appeal Board upheld the Panel’s rulings of breaches of Clauses 7.2 and 7.3. The appeal on this point was unsuccessful.
The advertisement featured the claim ‘Olmetec 20mg delivers more potent BP reduction than… candesartan 8mg’. A footnote stated that the medicines had been compared at their usual maintenance dose. The Appeal Board noted the dose for Olmetec was the optimal dose and the candesartan dose was the starting dose and usual maintenance dose. The Appeal Board considered that in practice such doses would be considered comparable. In this particular instance the Appeal Board considered that the basis of the comparison was clear. The Appeal Board ruled no breaches of Clauses 7.2 and 7.3 of the Code. The appeal on this point was successful.
* * * * *
During its consideration of this case, the Appeal Board noted that the Olmetec SPC stated that the recommended starting dose was 10mg once daily. In patients whose blood pressure was inadequately controlled at this dose, the dose might be increased to the optimal dose of 20mg once daily. Further, according to the SPC, the antihypertensive effect of Olmetec was substantially present within 2 weeks of initiating therapy and maximal by about 8 weeks after initiating therapy which should be borne in mind when considering changing the dose regimen. Thus 20mg was not the optimal dose for all patients, only for those whose blood pressure was inadequately controlled on 10mg. This was not made clear in the materials at issue.
Complaint received 31 May 2006
Case completed 28 September 2006


