
Reflections on Three Years at the PMCPA – Our Transformation Journey
Three years ago, I joined the PMCPA with the objective of creating a modern, sustainable organisation to deliver self-regulation now and into the future while continuing to deliver on our immediate priorities.
As we prepare to publish our 2024 annual report this week, I am reminded of how far we’ve come, but also that we have more to do and I am keen to build on the strong foundations we’ve established.
Alex Fell - Chief Executive, PMCPA
Reflections on Three Years at the PMCPA – Our Transformation Journey
09 September 2025
Developing a vision
We first needed a clear vision for self-regulation. We published this alongside refreshed branding in 2022 and our vision set out our aspiration for the organisation we wanted to become. We wanted the vision and brand to reflect both the PMCPA’s independence and the need to be externally facing and collaborative.
Our vision:
The PMCPA is the self-regulatory body for the pharmaceutical industry in the UK. By embedding high ethical standards and holding companies accountable for compliance, we provide confidence to patients and the public who rely on prescribed medicines, including vaccines.
We will do this by:
• Engaging and empowering companies by providing training and guidance on the ABPI Code of Practice for the Pharmaceutical Industry
• Ensuring high standards are upheld through a timely, robust, independent and transparent complaints system
• Using the benefit of self-regulation to ensure the ABPI Code and guidance react to changes in the environment and reflect latest industry practices.
Investing in the organisation
Across the UK, many regulatory bodies report increased volumes of complaints in recent years. This trend has impacted the PMCPA, exacerbated by the loss of 70+ years of experience shared between three individuals who left the PMCPA between 2022 and 2023.
To be fit for the future, we needed to invest in our people and our organisation, recruit effectively, and find new approaches to process complaints in a timely way. While much of this change is not visible externally, it has been critical to set ourselves up for long-term success.
It’s all about the people
Plain and simple, the PMCPA was under-resourced when I joined in 2022. For us to achieve our goals, we needed to substantially grow. To do that, we also needed to create an environment where we could attract, develop and retain talented individuals. As a self-regulatory body, it is important that we achieve the right balance of pharmaceutical industry experience, ABPI Code expertise, and experience and skills from other relevant backgrounds – this ensures we maintain our independence and bring in fresh thinking. The expansion in panel members has allowed us to adapt how we approach cases, matching expertise and experience to cases whilst allowing the space for training and development. We also wanted to reduce reliance on any one individual to help future-proof the organisation.
To do this, we have:
• Formalised job roles and career paths within the PMCPA
• Integrated a new team with diverse skillsets
• Invested in and promoted key talent within the team to lead many of our change initiatives
• Created a formal induction and training programme for new starters.
Modernising infrastructure and process
All parties involved in the complaints procedure trust us with highly confidential information – whether it’s a complainant’s identity or the confidential enclosures provided to us so we can assess a case.
During 2024, we implemented a new case management system that enables real-time reporting on our caseload, manages team allocation to cases and (as we add more data) will enable improved reporting on trends. We have already seen the benefits in the way we’re able to easily prioritise higher risk cases, allocate members of the Panel to work on each case, and identify any cases that might be amalgamated. We also implemented a board management system to manage the work of the Appeal Board. This has improved our data security, as well as enhancing the user experience.
We also needed to modernise our internal governance and procedures to effectively onboard new team members and ensure consistency in how we work. We developed a new digital site detailing step-by-step instructions for all aspects of the complaints procedure – from the case preparation stage through to Panel work, appeals and case reporting.
Seeing the impact
So, how are we doing? We often ask ourselves that and have set ourselves ambitious targets.
Operating the complaints procedure
Having taken the decision to invest in the organisation and infrastructure and improve the efficiency of how we work, I was confident that, over time, we would increase the number of rulings issued which, in turn, would reduce the backlog of complaints.
- Since Q1 2024, the number of members of the Code of Practice Panel has increased from 5 to 9 (FTE).
- We have seen a corresponding increase in the number of rulings issued per quarter.
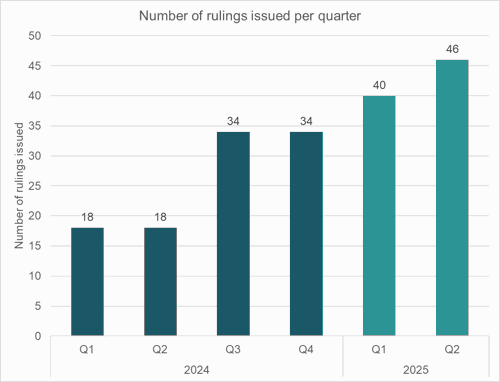
- The increased volume of cases processed in 2025 has been such that the number of open cases has reduced from 155 on 1 January 2025 to 94 by 31 July 2025.
It has been reassuring to see that the Panel has continued to have a good record, with 97% of rulings either accepted by the parties or upheld on appeal.
We acknowledge the progress made but are determined to maintain the momentum to reduce the time taken to adjudicate upon complaints, while maintaining the quality of our output.
Embedding and enhancing the ABPI Code of Practice
I am pleased with changes we made in the 2024 ABPI Code of Practice. We made major updates to Clause 12, including being one of only two countries in Europe at the time to introduce the use of QR codes on certain promotional materials to enable direct access to the up-to-date prescribing information.
We received so many comments during public consultation that we weren’t able to implement everything we wanted but are determined to do so in the future.
In 2025, our focus is on guidance to help embed the Code. We recently published guidance on Clauses 3.1 and 11 to provide practical interpretation of the Code and advice on the governance of pre-licence activities.
Other ongoing focus areas include a planned update to the PMCPA’s social media guidance, which includes a drive for consistency across Europe, and a new guidance document covering the use of package deals.
To help embed the Code, we launched a new e-learning platform to host on demand webinars and an e-learning module on the ABPI Code, which we added to in 2025 with more transparent information about the PMCPA audit process. In the last 12 months we also welcomed over 400 delegates to ‘Code in a day’ training days.
I was particularly proud that in May this year we held our third annual ‘ABPI Code for patient organisations’ training day, an initiative we started in collaboration with the ABPI in 2023.
Looking ahead, we expect that the EFPIA Code will be updated in the next couple of years and we are closely monitoring the potential changes and planning how the ABPI Code will adapt to ensure the UK is aligned with the rest of Europe. We want to use the next Code update to clarify and simplify a number of areas.
Improving transparency
In 2024, we changed the format of our case reports by including the complaint and company response in full (rather than paraphrasing). This has simplified the process of producing the reports and provides greater transparency. We have plans to review the format and structure of our case reports to further increase efficiency, improve readability and make learnings for industry more accessible.
From the 2024 annual report, the PMCPA now reports on the action taken on all complaints received and all case preparation manager decisions – not just those complaints which were progressed to a pharmaceutical company for a response.
The changes to the PMCPA Constitution and Procedure are explained here with many themed around simplification and increasing transparency. For example, the introduction of new provisions such as ‘set aside for procedural error’ provide a transparent and fair way of dealing with any issues that arise during the complaints procedure. The new abridged complaints procedure helps us ensure a proportionate response, allowing us to better prioritise our time.
What have we learned?
There have been many lessons along the way… my top three have been:
- Prioritisation is critical. We’re still a relatively small organisation (compared to other UK regulators), but we have big ambitions. We can’t do everything at once and so creating a roadmap for change and delivering the transformation needed in a sustainable way is critical as we maintain business continuity and the quality of our work product.
- Change is non-negotiable. The world is changing around us and, internally, the PMCPA could no longer rely on long-serving employees simply ‘knowing what to do’. We have made bold decisions to get the right team and infrastructure in place; we also recognised that doing nothing was not an option. We continuously listen to feedback, monitor the impact of any changes and adjust accordingly.
- Nothing is achieved in a vacuum. It is critical that we continue to benefit from the diverse experiences and expertise available to us. In my first few months in the role, I spent substantial time listening to and learning from a cross-section of stakeholders, before acting. I was especially grateful to learn from a highly skilled PMCPA team. Our colleagues at the ABPI provide invaluable support across multiple areas including IT, communications, finance and team development. While impossible to name everyone, the support and guidance from our counterparts at the MHRA has been, and continues to be, vital.
Looking to the future of self- regulation
Importantly, we now have the stability to take proactive steps to achieve our vision, to move from a largely reactive organisation to a more proactive approach to compliance. Self-regulation of the pharmaceutical industry remains a privilege and one we are determined to maintain.
The changes we made to the PMCPA Constitution and Procedure were important in that they strengthened both the independence of the PMCPA and procedural fairness to safeguard the interests of all parties. The new overriding objective provides a framework for making effective decisions whilst ensuring all parties act to protect patient safety.
We expect to start a public consultation on potential changes to the PMCPA Constitution and Procedure in early 2026. This will include proposals to further strengthen and simplify the complaints procedure, as well as a review of the effectiveness of sanctions for non-compliance with the Code. We will also consult on proposals to evolve our approach towards proactive monitoring and scrutiny of activities.
We also plan to offer greater support to companies that are new to the UK and/or self-regulation. Our ambition is that every eligible pharmaceutical company operating in the UK signs up to the self-regulatory framework and its provisions such as disclosure of payments to healthcare professionals, and certification of materials which help build public confidence in our industry through transparency and high standards.
As a team, we are energised by the challenges ahead and keen to listen and learn from our stakeholders along the way. I’m proud of what we’ve achieved in my first three years at the PMCPA and enjoy working in such a dedicated, resilient and talented team.
- Alex
